ANTHRAX VACCINE (AVA)
Documents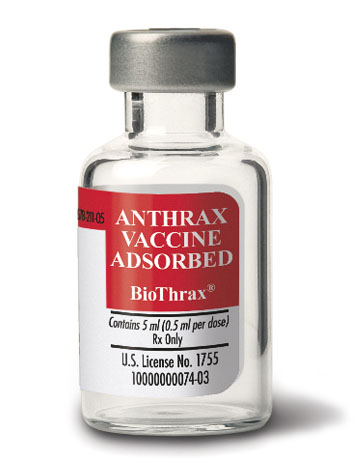
AVA Related MMQC Messages
Policies/Messages
NSN information
Anthrax vaccine has the following characteristics:
- National Stock Number (NSN): 6505-01-399-6828
- Unit of Issue: Ten 0.5ml doses per 5ml multidose vial (6.1ml +/- 0.2ml)
- National Drug Code (NDC): 64678-0211-05
- Storage: Store product at 2 to 8 degrees C (35.6 - 46.4 degrees F) DO NOT FREEZE
- Acquisition Advice Code: A
The following is the preferred list of ancillary supplies for the administration of anthrax vaccine, adsorbed.
NSN ITEM U/I
(NOTE: It is important to use these types of syringes ONLY as other types may affect the amount of vaccine which can be drawn from vial.)
Activities may substitute alternate ancillary supplies based on local availability and clinician preference so long as selected methods delivers a intramuscular injection dose of 0.5ml of anthrax vaccine, adsorbed. Anthrax vaccine may be administered by the subcutaneous route (for example, in persons who are at risk for hematoma formation following intramuscular injection.)
ANCILLARY SUPPLY EXPENSES ARE THE RESPONSIBILITY OF USING ACTIVITY.
AVA Related Links
Manufacturer